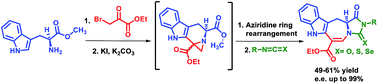
A facile and efficient synthesis of novel oxo, thio and seleno hydantoin fused tetrahydroazepino [4,5-b]indoles was reported. A naturally occurring iboga class alkaloid inspired seven-membered azepino[4,5-b]indole ring was synthesized as a new scaffold through Pictet–Spengler reaction followed by skeletal rearrangement of the aziridine ring. To improve the efficiency of the synthetic route, the double bond of the rearranged olefinic product 5 was reduced and a privileged hydantoin moiety was constructed on the core system through urea formation using a variety of isocyanates, isothiocyanates and isoselenocyanates followed by intramolecular cyclization to incorporate elements of diversity. The regeneration of the double bond of intermediate 9 afforded hydantoin-fused tetrahydroazepino [4,5-b]indoles.