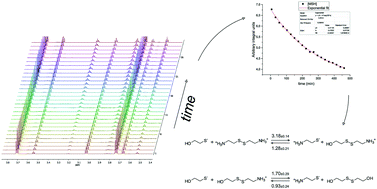
Microscopic rate constants were introduced and determined to quantify thiolate–disulfide kinetics of biological significance. The highly composite, codependent acid–base and redox processes of thiols could so far be characterized only in terms of apparent equilibrium and rate constants. A tailor-made evaluation method is elaborated with the use of quantitative NMR measurements to follow the kinetics of the forward and reverse reactions between mercaptoethanol and cystamine. The microscopic rate constants, which are new, pH-independent parameters, correlate nicely with the respective reactant structures and chemical characteristics. The reported rate constant values also allow a better understanding and prediction of thiolate–disulfide redox transitions.