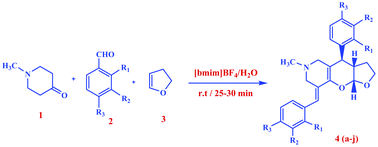
3,5-Diarylidene-1-methyl-piperidin-4-one derived in situ from N-methylpiperidinone and benzaldehyde undergoes intermolecular [4 + 2] hetero-Diels–Alder reactions with 2,3-dihydrofuran in [bmim]BF4–H2O (1 : 1) to afford furopyranopyridine derivatives in high to quantitative yields. Heterodienes, 3,5-diaryldiene-1-methylpiperidin-4-one an electron deficient oxadiene added to 2,3-dihydrofuran an electron rich dienophile because of an inverse electron demand, yielding furopyranopyridines with a high atom economy.