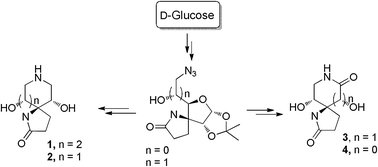
Synthesis of a new class of iminosugars 1–4 has been reported. The Jocic–Reeve and Corey–Link approach with α-D-glucofuranos-3-ulose 5 afforded 3-azidoaldehyde 7 that was converted to the γ-lactam 9. Reductive aminocyclisation and Schmidt–Boyer reactions were used to get spiro-iminosugars 1–4 which showed selective and potent glycosidase inhibitory activities. Molecular docking studies support the activity data.