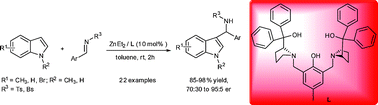
The asymmetric Friedel–Crafts amidoalkylation of indoles with aryl aldimines could be efficiently catalyzed by Trost's bis-ProPhenol dinuclear zinc complexes to attain 3-indolyl methanamine derivatives in good to excellent yields (85–98%) with moderate to high enantiomeric ratios (from 70 : 30 up to 95 : 5 er). Remarkably, this approach provides efficient access to enantiomerically enriched 3-indolyl methanamines, which avoids the formation of the undesirable bis- and tris(indolyl)methanes (BIMs and TIMs) byproduct.