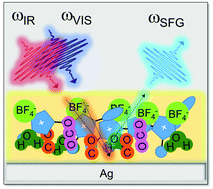
We used vibrational sum-frequency generation spectroscopy (SFG) to investigate low-overpotential CO2 reduction on a polycrystalline Ag electrode using room temperature ionic liquid (RTIL), 1-ethyl-3-methylimidazolium tetrafluorborate (EMIM-BF4) electrolyte mixtures with 0.3 mol%, 45 mol% and 77 mol% water. Adding water dramatically increases CO2 reduction efficiency up to 87.5 mol%. We found added water reduces the (negative) threshold potential for CO2 reduction from −1.33 V to −0.9 V. Added water also moved the potentials of the nonresonant (NR) SFG minima and caused the CO Stark shift to increase in concert with the reduction threshold. In previous work (N. García Rey and D. D. Dlott, J. Phys. Chem. C, 2015, 119, 20892–20899), with nearly-dry RTIL electrolyte (0.3 mol% water), we concluded a potential-driven structural transition of RTIL in the double layer controlled CO2 reduction. At lower water concentrations, where CO2 reduction was less efficient, CO product appeared primarily on Ag atop sites. At higher water concentrations where CO2 reduction efficiency was greater, adsorbed CO was observed on multiply-bonded sites, which are likely more efficient catalytic sites.