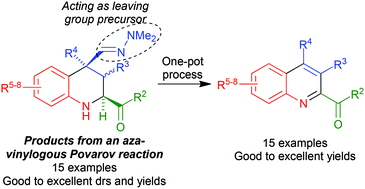
The aza-vinylogous Povarov reaction between α-ketoimines and α,β-unsaturated dimethylhydrazones in the presence of indium trichloride affords 2-acyl-4-alkyl-4-dimethylhydrazonomethyl-1,2,3,4-tetrahydroquinolines with good cis/trans diastereoselectivities. These compounds were readily transformed into highly substituted 2-acylquinolines using a two-reaction sequence involving the oxidative generation of a C-4 nitrile group, followed by its elimination under thermal conditions. Alternatively, a one-pot protocol based on the reaction of hydrazones with magnesium monoperoxyphthalate hexahydrate in refluxing methanol afforded the target 2-acylquinolines in good to excellent yields. This methodology was also extended to the preparation of 2-arylquinolines.