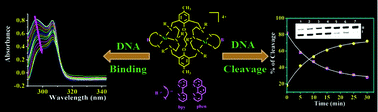
A new class of macrobicyclic dinickel(II) complexes [Ni2L1,2B](ClO4)4 (1–6), where L1,2 are polyaza macrobicyclic binucleating ligands, and B is a N,N-donor heterocyclic base (viz. 2,2′–bipyridine (bipy) and 1,10–phenanthroline (phen)) are synthesized and characterized. The redox, catalytic, DNA binding and DNA cleavage properties were studied. They exhibit two irreversible waves in the cathodic region around Epc = −0.95 V and Epa = −0.85 V vs. Ag/Ag+ in CH3CN–0.1 M TBAP, respectively. The first order rate constants for the hydrolysis of 4-nitrophenylphosphate to 4-nitrophenolate by the dinickel(II) complexes 1–6 are in the range from 3.36 × 10−5 to 10.83 × 10−5 Ms−1. The complexes 3 and 6 show good binding propensity to calf thymus DNA giving binding constant values (Kb) in the range from 3.08 × 105 to 5.37 × 105 M−1. The binding site sizes and viscosity data suggest the DNA intercalative and/or groove binding nature of the complexes. The complexes display significant hydrolytic cleavage of supercoiled pBR322DNA at pH 7.2 and 37 °C. The hydrolytic cleavage of DNA by the complexes is supported by the evidence from free radical quenching and T4 ligase ligation. The pseudo Michaelis–Menten kinetic parameters kcat = 5.44 × 10−2 h−1 and KM = 6.23 × 10−3 M for complex 3 were obtained. Complex 3 also shows an enormous enhancement of the cleavage rate, of 1.5 × 106, in comparison to the uncatalysed hydrolysis rate (k = 3.6 × 10−8 h−1) of ds-DNA.