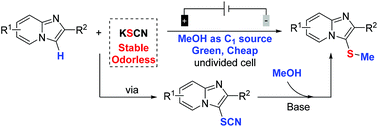
The electrochemical-induced regioselective C-3 thiomethylation of imidazopyridines has been initially accomplished via a three-component cross-coupling strategy using thiocyanate as the sulfur source and methanol as the methyl reagent. This protocol provides a green method for the thiomethylation of imidazopyridines without the need for any exogenous oxidants and metal catalysts under room temperature conditions. A wide range of functional groups were tolerated to produce regioselective C-3 thiomethylated products in high yields. Importantly, such an electrochemical-oxidation-induced thiomethylated reaction could be easily scaled up with excellent efficiency.