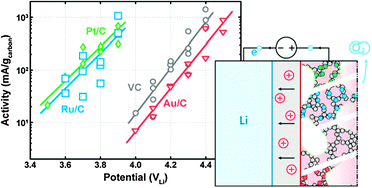
The oxidation kinetics of Li2O2 was studied in a carbonate-free electrolyte using electrodes consisting of non-catalyzed and catalyzed Vulcan carbon (VC) and chemically synthesized Li2O2 particles. VC and Au nanoparticles supported on VC (Au/C) were fairly inactive for catalyzing the oxidation of Li2O2, where oxidation currents greater than 10 mA gcarbon−1 were found only at voltages equal to and greater than 4.0 V vs. Li (VLi). Pt and Ru nanoparticles supported on VC (Pt/C and Ru/C) could significantly increase the kinetics of Li2O2 oxidation, where Li2O2 could be removed largely at voltages below 4 VLi. In addition, Pt/C and Ru/C showed quick initiation of Li2O2 oxidation in contrast to VC and Au/C.