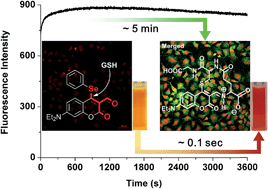
A phenyl-selenium-substituted coumarin probe was synthesized for the purpose of achieving highly selective and extremely rapid detection of glutathione (GSH) over cysteine (Cys)/homocysteine (Hcy) without background fluorescence. The fluorescence intensity of the probe with GSH shows a ∼100-fold fluorescent enhancement compared with the signal generated for other closely related amino acids, including Cys and Hcy. Importantly, the substitution reaction with the sulfhydryl group of GSH at the 4-position of the probe, which is doubly-activated by two carbonyl groups, occurs extremely fast, showing subsecond maximum fluorescence intensity attainment; equilibrium was reached within 100 ms (UV-vis). The probe selectivity for GSH was confirmed in Hep3B cells by confocal microscopy imaging.