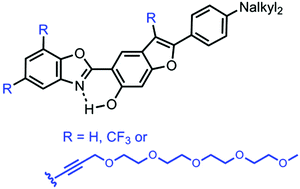
This article describes the multi-step synthesis of 2-(2′-hydroxybenzofuran)benzoxazole (HBBO) derivatives functionalised with one to three oligo(ethylene glycol) (OEG) chains with the goal to allow a good vectorization in aqueous media. Benzoxazole dyes are well-known organic fluorophores exhibiting excited-state intramolecular proton transfer (ESIPT) emission. The insertion of these highly hydrophilic OEG chains allows a good solubilization of these dyes in a wide range of solvents of different polarity including PBS buffer/DMSO (95/5) and water, for one of them. The photophysical properties of these ESIPT emitters in solution, and also in the solid-state, as doped as 1 wt% in poly(methylmethacrylate) (PMMA) or polystyrene (PS) films, are discussed. Specifically, the presence of an OEG chain at the 3-position of the benzofuran ring was found to sizeably enhance the fluorescence intensity of the ESIPT emission in the solution state.