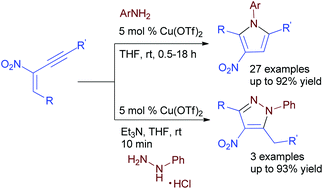
Various tetrasubstituted pyrroles/pyrazoles have been prepared from nitro-substituted 1,3-enynes with aromatic amines/hydrazines via a copper-catalyzed cascade aza-Michael addition, cyclization and aromatization at room temperature. This protocol is also effective for the synthesis of tetrasubstituted pyrazoles in high yields.