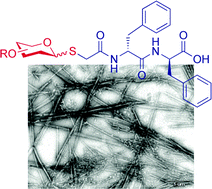
Diphenylalanine, a key building block for organic nanotechnology, forms discrete, rigid and hollow nanotubes that are assembled spontaneously upon their dilution from organic phase into aqueous solution. Here we report the efficient preparation of several S-linked glycosylated diphenylalanine analogues bearing different monosaccharide, di-saccharide and sialic acid residues. The self-assembly studies revealed that these glycopeptides adopted various structures and glycosylation could be a tool to manipulate the self-assembly process. Moreover, the solubility of these analogues was found to be much greater than diphenylalanine, which could open new applications based on these nanostructures.