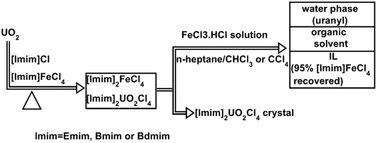
Imidazolium-based Fe-containing ionic liquids (ILs) can directly dissolve UO2 in the presence of their corresponding imidazolium chlorides without additional oxidants. The dissolution process follows pseudo first-order kinetics initially. Raman spectroscopic studies indicate that FeCl42− is the predominant reduction product after UO2 dissolution, and attenuated total reflection-Fourier transform infrared spectroscopy indicates that the UO22+ complex is the principal product in the ILs. The dissolved uranyl species can be successfully separated from the Fe-containing ILs via a combination of centrifugation and solvent extraction, and also, the Fe-containing ILs can be recovered easily. In conclusion, imidazolium-based Fe-containing ionic liquids in the presence of imidazolium chlorides could be used as effective and recoverable oxidants for the dissolution of UO2.