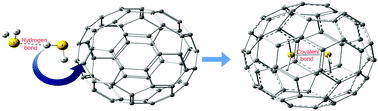
The present work investigates the effect of confinement on the hydrogen bonding interactions in H2S dimers. The interiors of different sized fullerenes (C60, C70, C84, and C120) have been used to model the effect of confinement. It was found that as the degree of confinement increases, the hydrogen bonding between H2S molecules disappears and sulphur–sulphur interactions appear. We obtained clear computational evidence that, inside C60, the H2S dimer is bound by a covalent bond between two sulphur atoms. It was also found that the strength of the S–S bond inside fullerenes is linked to the amount of charge transfer from the H2S dimer to the fullerene.