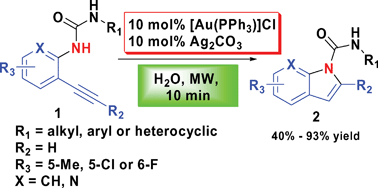
Using [Au(PPh3)]Cl/Ag2CO3-catalyzed 5-endo-digcyclization in water under microwave irradiation, we developed a fast and green route to prepare indole-1-carboxamides from N′-substituted N-(2-alkynylphenyl)ureas. The described method is tolerant to a variety of functional groups, including N′-aryl, alkyl, heterocyclic, various N-(substituted-2-ethynylphenyl) and N-(2-ethynylpyridin-3-yl)ureas and affords moderate to high yields of the desired products.