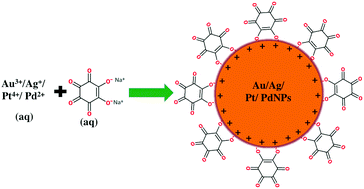
Sodium rhodizonate was used as a bifunctional reducing as well as stabilizing agent for the single step synthesis of gold (Au), silver (Ag), platinum (Pt), and palladium (Pd) nanoparticles (NPs) in water. Transmission electron microscopy analysis revealed that Pt, Au, Ag, and PdNPs have average core diameters of about 2, 8, 26, and 39 nm, respectively. The ability of these nanoparticles towards the catalytic reduction of 4-nitrophenol (4-NP) with sodium borohydride (NaBH4) and the dual-catalytic oxidation of formic acid followed by the reduction of methyl orange (MO) was studied. The apparent rate constants (kapp) of the catalytic reduction of 4-NP in the presence of Ag, Au, Pt, and PdNPs were calculated to be 2.1482, 1.1167, 1.088 × 10−1, and 1.65 × 10−2 min−1, respectively. However, for the dual-catalytic oxidation of formic acid followed by the reduction of MO, the kapp values were calculated to be 4.145, 1.25 × 10−2, 6.7 × 10−3, and 9.0 × 10−5 for the Pt, Pd, Au, and AgNPs, respectively.