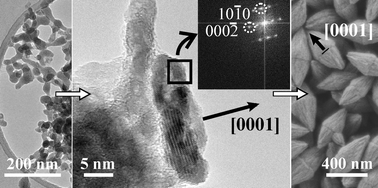
In this study we report on the growth behavior of zinc oxide in the presence of different concentrations of silicon. We performed reactions in a continuous tubular reactor in aqueous and ethanolic-aqueous media at different reaction temperatures and for different residence times. It was found that the zinc oxide particles grew from the aqueous medium through the crystallization of amorphous zinc oxide units via different growth stages, i.e., plate-like particles, double-plate-like particles and ellipsoidal particles. The Zn/OH atomic ratio and, consequently, the pH control the particle size and the morphology (i.e., the inclusions on the particle surfaces). The presence of silicon in the reaction solution influences the particle morphology (the formation of planar defects), the particle growth stages (the double-plate-like particles) as well as the particle growth rate. By shifting the UV-VIS absorption maxima of the reaction suspensions we showed that an increased silicon concentration and a decreased reaction temperature significantly retarded the growth rate of the zinc oxide.