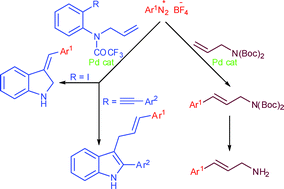
Novel palladium-catalyzed reactions of arenediazonium tetrafluoroborates with N,N-diprotected allylamines are presented. The reaction of arenediazonium tetrafluoroborates with N,N-(Boc)2 allylamine allows for an easy approach to cinnamylamines whereas using 2-alkynyl-N-(allyl)trifluoroacetanilides and 2-iodo-N-(allyl)trifluoroacetanilide the reaction provides a useful tool for appending indole rings to aniline fragments.