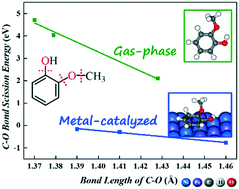
We examine the initial hydrodeoxygenation (HDO) of guaiacol on bimetallic NiFe(111) and PtFe(111) using the density functional theory. Our results show that on NiFe(111), direct Caryl–O bond breaking and dehydrogenation are preferred over hydrogenation, while on PtFe(111), hydrogenation and dehydrogenation are preferred over Caryl–O bond breaking. Catechol is the major product of guaiacol HDO on both Fe-alloyed surfaces via dehydrogenation of methoxy (OCH3) followed by O–CH2 bond scission being promoted by oxophilic Fe alloying. In comparison, the removal of the oxo functional group of guaiacol (i.e., Caryl(α)–OH, Caryl(β)–OCH3 and Caryl(β)O–CH3 bond breaking) on both Fe-alloyed surfaces is more facile energetically than those on monometallic Ni(111) and Pt(111) owing to oxophilic Fe active surface sites. It is confirmed that the C–O bond length of adsorbed intermediates can serve as a good descriptor for predicting the C–O bond scission reactivity of the lignin-derived phenolic compounds on metal surfaces depending on C–O bond scission types.