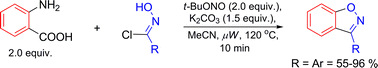
An efficient protocol for the synthesis of a range of 1,2-benzisoxazoles using an improved 1,3-dipolar cycloaddition of nitrile oxides and benzyne is described. Key to the procedure is the in situ generation of the reactive nitrile oxide and benzyne reactants simultaneously.