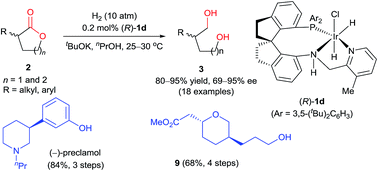
We report a protocol for the highly efficient iridium-catalyzed asymmetric hydrogenation of racemic α-substituted lactones via dynamic kinetic resolution. Using Ir-SpiroPAP (R)-1d as a catalyst, a wide range of chiral diols were prepared in a high yield (80–95%) with a high enantioselectivity (up to 95% ee) under mild reaction conditions. This protocol was used for enantioselective syntheses of (−)-preclamol and a chiral 2,5-disubstituted tetrahydropyran.