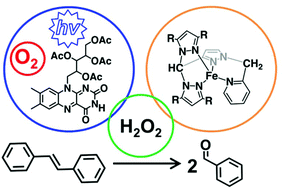
Attachment of a 2-methylpyridyl group onto the unique 1-nitrogen atom on nitrogen-confused C-scorpionates with either pyrazol-1-yl or 3,5 dimethylpyrazol-1-yl donors gives two new cis-directing tetradentate-N4 ligands (L and L*). The complexes [(L or L*)Fe(CH3CN)2](BF4)2 (1 or 2) were prepared, fully characterized, and investigated for their ability to catalyse the oxidative cleaveage of trans-stilbene in CH3CN. Complexes 1 and 2 are capable of catalysing stilbene cleavage when H2O2 is used as an oxidant but up to six different products are formed, with C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C cleavage products (benzaldehyde and benzoic acid) dominating over four products of oxygen transfer. Catalytic amounts of 1 or 2 enhance the ability for the organic photocatalyst riboflavin tetraacetate to use atmospheric oxygen and blue light irradation (450–460 nm) to selectively cleave stilbene to benzaldehyde. However, when benzaldehyde oxidizes further to benzoic acid, the iron species begin giving increasing amounts of stilbene oxygenation products.
C cleavage products (benzaldehyde and benzoic acid) dominating over four products of oxygen transfer. Catalytic amounts of 1 or 2 enhance the ability for the organic photocatalyst riboflavin tetraacetate to use atmospheric oxygen and blue light irradation (450–460 nm) to selectively cleave stilbene to benzaldehyde. However, when benzaldehyde oxidizes further to benzoic acid, the iron species begin giving increasing amounts of stilbene oxygenation products.