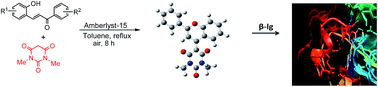
An efficient method for synthesis of 1,3-dimethyl-5-(2-phenyl-4H-chromen-4-ylidene)pyrimidine-2,4,6(1H,3H,5H)-triones has been developed by treatment of 2-hydroxychalcones and 1,3-dimethylbarbituric acid in refluxing toluene in the presence of amberlyst-15 as catalyst in air. The process involves the occurrence of a domino sequence of Michael addition, cyclization and aerial oxidation. The compounds synthesized showed an interesting property of free rotation around the bond linking the two heterocyclic moieties and three of them were found to show binding property with the milk protein β-lactoglobulin (β-lg). DFT and docking (with β-lg) studies of one of the compounds have been done.