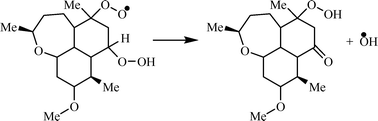
The kinetic schemes of intramolecular reactions of five analogs of artemisinin were built. The method of intersecting parabolas was used for the calculation of activation energies and rate constants of each elementary step of these schemes. The competition between monomolecular and bimolecular free radicals was taken into account. It was evidenced that the intramolecular oxidation of these compounds proceeds as a cascade of consecutive free radical reactions with the formation of hydroperoxide groups. The latter decompose via reactions with the Fe(II) complexes generating free radicals. Among the radicals formed, the hydroxyl radical was proved to play the key role. A correlation between the yield of hydroxyl radicals nOH and antimalarial activity of compounds (IC50) was observed. The dependence of index IC50 on nOH is linear in the logarithmic coordinates: ln[IC50(Artemisinin)/IC50(Compound)] = −14.10 + 3.85 × nOH. The proposed scheme explains and demonstrates a strong dependence of the antimalarial effectiveness of a drug on the chemical structure.