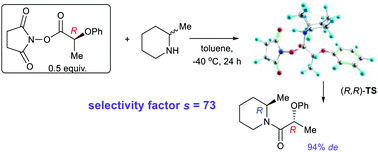
The diastereoselective acylation of a number of racemic methyl-substituted cyclic alkylamines with active esters of 2-phenoxypropanoic acid was studied in detail. The ester of (R)-2-phenoxypropanoic acid and N-hydroxysuccinimide was found to be the most selective agent. The highest stereoselectivity was observed in the kinetic resolution of racemic 2-methylpiperidine in toluene at −40 °C (selectivity factor s = 73) with the predominant formation of (R,R)-amide (93.7% de). To explain the observed stereoselectivity, DFT modelling of the transition states in the reactions of the title acylating agent with 2-methylpiperidine and 2-methylpyrrolidine was performed. The calculated values were in good agreement with experimental data. It has been demonstrated that the acylation proceeds via a concerted mechanism, in which the addition of an amine occurs simultaneously with the elimination of the hydroxysuccinimide fragment. The high stereoselectivity of the (R,R)-amide formation is largely ensured by the lower steric hindrances in the transition states as compared to the formation of (R,S)-amide.