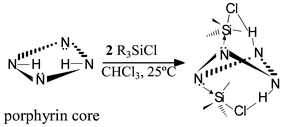
Interaction of various molar ratios of meso-tetraphenylporphyrins with two different trialkylsilyl chlorides in chloroform only afforded l∶2 molecular complexes at room temperature. Spectral properties closely corresponded to those of diprotonated meso-tetraphenylporphyrin, strongly suggesting similar pseudo tetrahedrally distorted porphyrin core structures with σ-bonding from two pyrrolenine nitrogen donors to the silicon centers and two hydrogen bonds between two pyrrole NH⋯ and ⋯ClSi groups from above and below the mean plane of the porphyrins with nearly trigonal bipyramidal coordination geometries for silicon centers, with alkyl groups located at the equatorial sites.