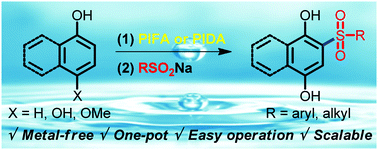
A one-pot method towards sulfonylated hydroquinones/naphthalenediols in an aqueous medium has been developed with up to 97% yield. The whole reaction requiring no transition-metal catalysts could proceed smoothly with hypervalent iodine compounds as the oxidant. Both naphthols and phenols were viable with inexpensive and readily available sodium sulfinates as the sulfonylation reagents under an ambient atmosphere. This procedure is scalable, and the products could be easily obtained without column chromatography isolation.