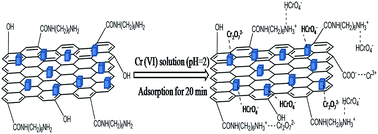
A one-step solvothermal method was developed to prepare nearly cubic ZnFe2O4 nanoparticles loaded on 1,6-hexanediamine-functionalized reduced graphene oxide (HDA–RGO–ZnFe2O4) for fast removal of Cr(VI). The materials were characterized by transmission electron microscopy, Fourier-transform infrared spectroscopy, X-ray diffraction, Raman, and vibrating sample magnetometry. Experimental results indicated that Cr(VI) adsorption by HDA–RGO–ZnFe2O4 is strongly pH dependent and the adsorption equilibrium could be reached within 12 min. The kinetic adsorption of different initial concentrations can be accurately described by the pseudo-second-order model. The overall rate process was apparently influenced by external mass transfer and intraparticle diffusion. The Langmuir isotherm model is consistent with the experimental data at different temperatures and the maximum adsorption capacity was determined to be 172.4 mg g−1. Moreover, the thermodynamic parameters indicated that the adsorption process was spontaneous and endothermic. The adsorption mechanism and desorption experiments were also studied. After adsorption, the HDA–RGO–ZnFe2O4 could be quickly separated from the media by an external magnetic field and the adsorption capacity can remain up to 82% after five times of usage. The results implied that HDA–RGO–ZnFe2O4 is a promising adsorbent for the removal of Cr(VI).