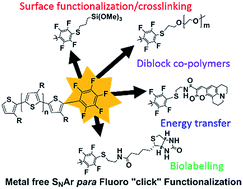
A convenient method of introducing pentafluorobenzene (PFB) as a single end-group in polythiophene derivatives is reported via in situ quenching of the polymerization. We demonstrate that the PFB-group is a particularly useful end-group due to its ability to undergo fast nucleophilic aromatic substitutions. Using this molecular handle, we are able to quantitatively tether a variety of common nucleophiles to the polythiophene backbone. The mild conditions required for the reaction allows sensitive functional moieties, such as biotin or a cross-linkable trimethoxysilane, to be introduced as end-groups. The high yield enabled the formation of a diblock rod-coil polymer from equimolar reactants under transition metal-free conditions at room temperature. We further demonstrate that water soluble polythiophenes end-capped with PFB can be prepared via the hydrolysis of an ester precursor, and that such polymers are amenable to functionalization under aqueous conditions.