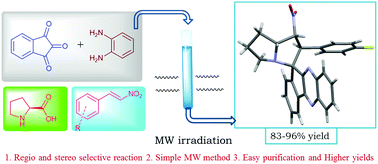
In this study, we report an efficient four-component cascade protocol to afford spiro indeno[1,2-b]quinoxaline-11,3′-pyrrolizines via the condensation of ninhydrin, phenylenediamine, proline, and nitrostyrene derivatives under microwave irradiation and classical conditions. The 1,3-dipolar cycloaddition reaction was found to proceed in a highly regio- and stereoselective manner. This methodology exemplifies the green chemistry protocol for the preparation of spiro-pyrrolizines. In addition, all the synthesized compounds were screened for AChE inhibitory activity and 6 out of 21 compounds depicted significant activity in the low micromolar IC50 range.