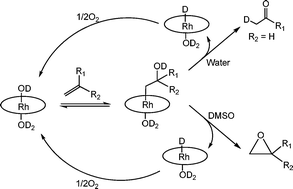
This article reports on the selective oxidation of unactivated alkenes to ketones and epoxides through the intermediacy of β-hydroxyalkyl rhodium porphyrin complexes which are formed by reactions of terminal alkenes with tetra(p-sulfonatophenyl)porphyrin rhodium(III) complex. The β-hydroxyalkyl rhodium porphyrin complexes in water undergo β-C–H elimination to produce ketones in aqueous pH 9.0 solutions and O–H deprotonation in KOH/DMSO solutions resulting in the rapid and quantitative intramolecular nucleophilic displacement to form 1,2-epoxyalkanes.