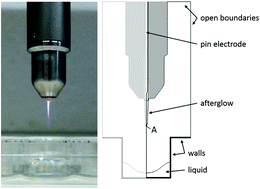
Plasma-treated liquids have great potential for biomedical applications. However, insight into the underlying mechanisms and the exact chemistry is still scarce. In this study, we present the combination of a 0D chemical kinetics and a 2D fluid dynamics model to investigate the plasma treatment of a buffered water solution with the kINPen® plasma jet. Using this model, we calculated the gas and liquid flow profiles and the transport and chemistry of all species in the gas and the liquid phase. Moreover, we evaluated the stability of the reactive oxygen and nitrogen species after plasma treatment. We found that of all species, only H2O2, HNO2/NO2−, and HNO3/NO3− are stable in the buffered solution after plasma treatment. This is because both their production and loss processes in the liquid phase are dependent on short-lived radicals (e.g. OH, NO, and NO2). Apart from some discrepancy in the absolute values of the concentrations, which can be explained by the model, all general trends and observations in our model are in qualitative agreement with experimental data and literature.