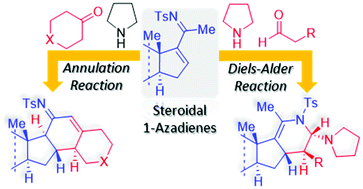
The chemical behavior of steroidal N-sulfonyl-1-azadienes toward carbonyl compounds, in the presence of pyrrolidine, is described. With aldehydes, these azadienes participate in hetero-Diels–Alder reactions with the in situ generated enamines. The stereoselectivity results from the approach of the dienophiles from the less hindered α-face of the steroid, with the pyrrolidine moiety endo and retention of the enamine trans geometry. This diastereoselective synthetic methodology led to a new class of chiral pentacyclic steroids. Interestingly, the studied steroidal scaffolds follow a different mechanistic pathway with cyclic ketones. They undergo a diastereoselective annulation reaction, under enamine catalysis, affording chiral hexacyclic steroids.