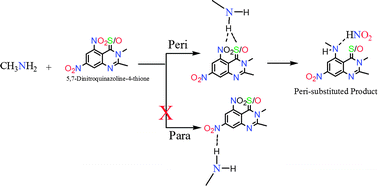
The microcosmic mechanism of nucleophilic aromatic substitution of the nitro groups of 5,7-dinitroquinazoline-4-one with methylamine has been investigated in gas phase as well as in solvent media within the formalism of density functional theory. The free energy profiles along the reaction route, structural parameters, charge analysis and frontier molecular orbitals provide sufficient evidence to conclude that no stable intermediates are formed during the reaction course and a concerted mechanism is followed in this nucleophilic substitution. The regioselectivity involved in the reaction is attributed to intramolecular hydrogen bonding (N–H–O![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C) that stabilises the peri-transition state. Similar to carbonyls, thiocarbonyls have also shown the regioselective attack of amines at the peri-position of 5,7-dinitroquinazoline-4-thione through intramolecular hydrogen bonding (N–H–S
C) that stabilises the peri-transition state. Similar to carbonyls, thiocarbonyls have also shown the regioselective attack of amines at the peri-position of 5,7-dinitroquinazoline-4-thione through intramolecular hydrogen bonding (N–H–S![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C).
C).