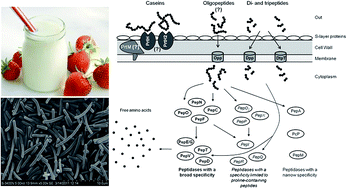
Three strains of Lactobacillus delbrueckii subsp. bulgaricus were selected for their proteinase properties in order to improve milk gel firmness. The respective proteinases were purified by ultra-filtration, anion exchange, and hydrophobic interaction chromatographies. The 3 purified proteinases were determined to have molecular masses of about 39, 40, and 52 kDa. The optimal activities of the purified enzymes occurred at pH 6.0 and 40 °C. They are metallopeptidases, activated by Fe2+, inhibited by Ba2+, Zn2+, Mn2+, Xi2+, Fe3+, Cu2+ and EDTA, and serine proteinases which are inhibited by PMSF.