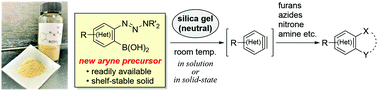
We report the development of o-triazenylarylboronic acids as new aniline-based aryne precursors. The readily available and shelf-stable solid precursors generate (hetero)arynes under remarkably mild conditions using silica gel as the sole reagent, which subsequently undergo reactions with a range of arynophiles. Furthermore, solid-state aryne reactions under solvent-free conditions were accomplished. Aryne generation proceeded via a dual activation mechanism, as rationalized using Jaffé's plot analysis based on Hammett constants.