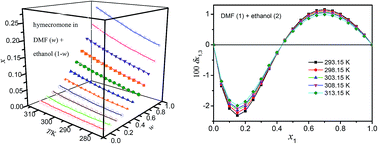
Solubilities of hymecromone in neat solvents of N,N-dimethylformamide (DMF), methanol, ethanol and n-propanol, and their binary mixed solvents of DMF + methanol, DMF + ethanol and DMF + n-propanol were determined using an isothermal dissolution equilibrium method within the temperature range from 278.15 K to 313.15 K under 101.1 kPa. They were correlated with the Jouyban–Acree, van't Hoff–Jouyban–Acree and Apelblat–Jouyban–Acree models obtaining relative average deviations (RAD) lower than 0.51% and root-mean-square deviation (RMSD) lower than 4.42 × 10−4. Positive values of the dissolution enthalpy illustrated that the dissolution process of hymecromone in these mixed solvents was endothermic. Furthermore, the preferential solvation parameters were derived by using the inverse Kirkwood–Buff integrals. The preferential solvation parameters (δx1,3) were negative in alcohol-rich mixtures but positive in compositions from 0.35 (0.43, 0.50) in the mole fraction of DMF to neat DMF.