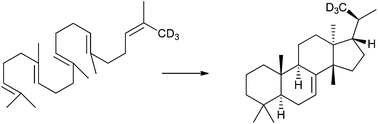
The squalene cyclase of Tetrahymena pyriformis cyclizes 2,3-dihydrosqualene to euph-7-ene. This was used as a model for euphane biosynthesis. D-ring formation was demonstrated to involve pre-chair folding through an experiment with 2,3-dihydro-5-oxasqualene. This requires that a non-least motion rotation (120°) of the C17–20 bond in the intermediate deoxydammaranyl cation occurs before the first 1,2-hydride shift. Truncated substrates relieved the hindrance associated with this rotation and permitted a least motion pathway. Several triterpenes were found to be minor products of the Tetrahymena cyclase.