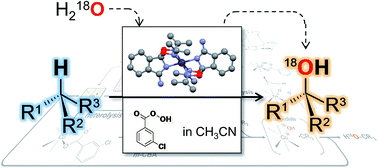
Two complexes with isoindole-core ligands of general formula [M{C6H4C(NH2)NC(ONCMe2)2}2](NO3)2 (M = Co for 1 and M = Ni for 2) were studied as catalysts for the mild stereoselective alkane oxidation with m-chloroperbenzoic acid (m-CPBA) as an oxidant and cis-1,2-dimethylcyclohexane (cis-1,2-DMCH) as a main model substrate. Complex 1 disclosed a pronounced activity, with high retention of stereoconfiguration of substrates (>98% for cis-1,2-DMCH) and highest cis/trans ratio of tertiary alcohols (products) of 56, under mild conditions. The best achieved yields of tertiary cis-alcohols were of 13.7 and 50.5%, based on the substrate (cis-1,2-DMCH) and the oxidant (m-CPBA) respectively. Kinetic experiments, high bond and stereoselectivity parameters, kinetic isotope effect of 7.2(2) in the oxidation of cyclohexane, and incorporation of 18O from H218O support the involvement of CoIV![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O high-valent metal–oxo intermediates as main C–H attacking species.
O high-valent metal–oxo intermediates as main C–H attacking species.