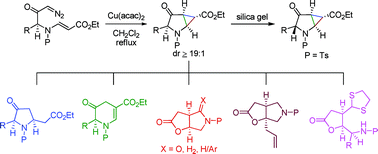
A new, highly stereoselective intramolecular cyclopropanation of vinylogous carbamates with carbenes in the presence of Cu(acac)2 as the catalyst has been developed for the construction of cyclopropapyrrolidinones. The ‘syn’ isomer of N-DAC can be converted to the ‘anti’ isomer by simple silica gel treatment. Regioselective cleavage of each of the cyclopropane bonds of these two acceptor substituted N-DACs led to a diverse array of azacycles.