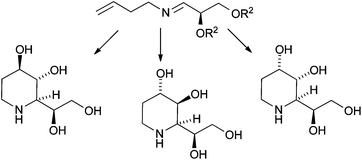
The stereoselective synthesis of D-fagomine, D-3-epi-fagomine, and D-3-epi-fagomine analogs starting from readily available D-glyceraldehyde acetonide has been achieved. The synthesis involves diastereoselective anti-vinylation of its homoallylimine, ring-closing metathesis, and stereoselective epoxidation followed by regioselective ring-opening or stereoselective dihydroxylation. The lack of a strong activity as glycosidase inhibitors of these compounds could be advantageous for their therapeutic use as chaperones.