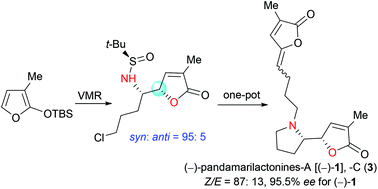
The synthesis of pandamarilactonines in high enantiopurity is challenging due to the configurational instability of the pyrrolidin-2-yl butenolide moiety present in these alkaloids. In an attempted asymmetric synthesis of pandamarilactonine-B (2), an unanticipated syn-diastereoselective (dr = 95 : 5) asymmetric vinylogous Mannich reaction (VMR) between 3-methyl-2-(tert-butyldimethylsilyloxy)furan 9b and the Ellman (RS)-N-tert-butanesulfinimine 10a was observed. The methyl group at C-3 of TBSOF has been shown to play the role of a switch of diastereoselection in the VMR. This work ended with a concise three-pot protecting-group-free total synthesis of (−)-pandamarilactonine-A. The puzzle about the configurational instability of the pyrrolidinyl butenolide skeletons has also been disclosed by controlled experiments and with the help of computation.