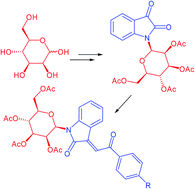
N-Glycosyl-3-alkylideneoxindoles, N-glycosylated 3-(2-oxo-2-arylethylidene)indolin-2-ones, were prepared by reaction of isatin-N-glycosides with substituted acetophenones. The biological activities of these new compounds revealed significant antitumor activity in melanoma human cell lines. Inhibition of cell proliferation and loss of cell viability were strongly enhanced by the combination with the death ligand TRAIL (TNF-related apoptosis-inducing ligand). The antitumor effects were related to the inhibition of the survival pathway of c-Jun and JNK2 (Jun N-terminal kinase).