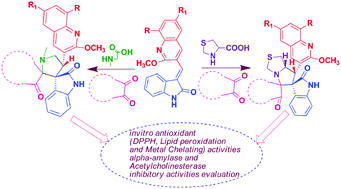
A regioselective synthesis of new mono and bisoxindoles containing bis spiro heterocyclic hybrids has been envisaged via 1,3-dipolar cycloaddition of azomethine ylides with the dipolarophile (E)-3-((2-methoxyquinolin-3-yl)methylene)indolin-2-one, obtained through the base catalyzed condensation of indolin-2-one with substituted 2-methoxyquinoline-3-carbaldehyde. The regiochemistry and stereochemistry of the synthesized products were characterized by 1D and 2D NMR techniques and single crystal X-ray diffraction studies. Many of the compounds were found to show in vitro antioxidant, antidiabetic and acetylcholinesterase inhibitory activities.