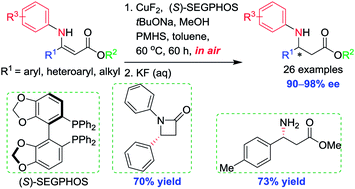
In the presence of the inexpensive and stable stoichiometric reductant polymethylhydrosiloxane (PMHS) as well as certain amounts of appropriate alcohol and base additives, the non-precious metal copper-catalyzed asymmetric 1,4-hydrosilylation of β-aryl or β-alkyl-substituted N-aryl β-enamino esters was well realized to afford a diverse range of N-aryl β-amino acid esters in high yields and excellent enantioselectivities (26 examples, 90–98% ee). This approach tolerated the handling of both catalyst and reactants in air without special precautions. The chiral products obtained have been successfully converted to the corresponding enantiomerically enriched β-lactam and unprotected β-amino acid ester, which highlighted the synthetic utility of the developed catalytic procedure.