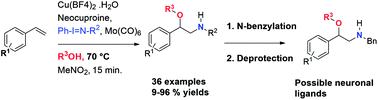
A regioselective, copper-catalyzed, one-pot aminoalkoxylation of styrenes using primary and secondary alcohols and three different iminoiodanes as alkoxy and nitrogen sources respectively, is reported. The β-alkoxy-N-protected phenethylamines obtained were used to synthesise β-alkoxy-N-benzylphenethylamines which are interesting new compounds that could act as possible neuronal ligands.