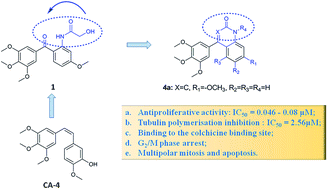
In the course of our search for novel antitumor agents, a series of cyclic combretastatin A-4 (CA-4) analogues bearing an amide group, A–B or B–C ring condensation, and C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C or C
C or C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) N bond in the B ring were designed, synthesized and identified as new microtubule inhibitors. The structure–activity relationship (SAR) studies showed that the hexa-cyclic compounds bearing B–C ring condensation, containing a C
N bond in the B ring were designed, synthesized and identified as new microtubule inhibitors. The structure–activity relationship (SAR) studies showed that the hexa-cyclic compounds bearing B–C ring condensation, containing a C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C bond in the B ring (4a) provided excellent antiproliferative activities at nanomolar concentrations against various cancer cell lines (IC50 = 46–80 nM). 4a inhibited tubulin assembly at a micromolar range (IC50 = 2.56 ± 0.15 μM) as evidenced by a molecular docking study, which revealed that 4a exerted tubulin polymerisation inhibitory activity by binding to the colchicine binding site of tubulin. Further molecular biology studies showed that 4a disrupted intracellular microtubule polymerisation and thus induced G2/M phase arrest and apoptotsis in A549 cells. Altogether, these results we obtained can guide the design of novel potent molecules for future development.
C bond in the B ring (4a) provided excellent antiproliferative activities at nanomolar concentrations against various cancer cell lines (IC50 = 46–80 nM). 4a inhibited tubulin assembly at a micromolar range (IC50 = 2.56 ± 0.15 μM) as evidenced by a molecular docking study, which revealed that 4a exerted tubulin polymerisation inhibitory activity by binding to the colchicine binding site of tubulin. Further molecular biology studies showed that 4a disrupted intracellular microtubule polymerisation and thus induced G2/M phase arrest and apoptotsis in A549 cells. Altogether, these results we obtained can guide the design of novel potent molecules for future development.