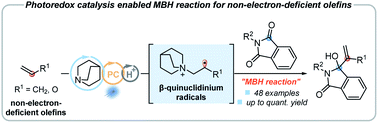
A strategy for overcoming the limitation of the Morita–Baylis–Hillman (MBH) reaction, which is only applicable to electron-deficient olefins, has been achieved via visible-light induced photoredox catalysis in this report. A series of non-electron-deficient olefins underwent the MBH reaction smoothly via a novel photoredox-quinuclidine dual catalysis. The in situ formed key β-quinuclidinium radical intermediates, derived from the addition of olefins with quinuclidinium radical cations, are used to enable the MBH reaction of non-electron-deficient olefins. On the basis of previous reports, a plausible mechanism is suggested. Mechanistic studies, such as radical probe experiments and density functional theory (DFT) calculations, were also conducted to support our proposed reaction pathways.